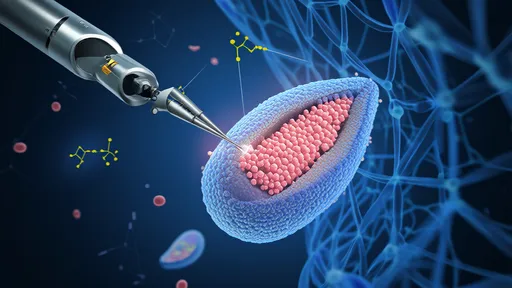
In a groundbreaking leap for regenerative medicine, scientists are pioneering mitochondrial transplantation therapy—a revolutionary approach that could repair damaged cells at their energetic core. Dubbed "nano-surgery for the cellular power plants," this technique involves extracting healthy mitochondria from donor cells and delivering them into compromised tissues, offering hope for conditions ranging from neurodegenerative diseases to heart failure. The implications are profound: by restoring the body’s ability to produce energy, researchers aim to reverse cellular decay and unlock new frontiers in treating age-related and genetic disorders.
The concept hinges on mitochondria’s role as the cell’s powerhouse. These tiny organelles generate adenosine triphosphate (ATP), the currency of cellular energy. When mitochondria malfunction due to mutations, oxidative stress, or aging, cells starve, leading to organ dysfunction. Traditional therapies often address symptoms rather than the root cause. Mitochondrial transplantation, however, targets the defect directly. Early experiments show transplanted mitochondria integrating into host cells, replenishing ATP production, and even improving survival rates in critical cases like ischemic heart tissue.
How does this microscopic feat work? The process begins with isolating viable mitochondria from healthy tissue—often a patient’s own or from matched donors. Using advanced techniques like centrifugation or microfluidics, researchers purify these organelles before injecting them into target areas. Some methods employ nanoparticles or viral vectors as delivery vehicles, ensuring precise placement. Once inside recipient cells, the new mitochondria fuse with existing networks, sharing genetic material and restoring metabolic function. Animal studies demonstrate reduced inflammation and improved tissue repair, sparking optimism for human trials.
One of the most promising applications lies in neurology. Neurodegenerative diseases like Parkinson’s and Alzheimer’s are marked by mitochondrial dysfunction in neurons. In lab models, transplanted mitochondria have been shown to migrate along neural pathways, rescuing dying cells and improving cognitive function. Cardiologists are equally intrigued: after heart attacks, mitochondrial grafts could rejuvenate oxygen-deprived myocardium, preventing scar formation. Even rare genetic disorders like Leigh syndrome, where mutated mitochondrial DNA cripples energy production, might one day be treated with this approach.
Despite its potential, the therapy faces hurdles. Ensuring mitochondrial compatibility is critical—mismatches could trigger immune rejection or metabolic chaos. Researchers are exploring autologous transplants (using a patient’s own mitochondria) or creating universal donor lines with edited DNA. Another challenge is scaling up delivery; while localized injections work for some organs, systemic distribution requires innovative carriers that evade the immune system. Ethical questions also arise, particularly regarding germline editing if mitochondrial DNA alterations are heritable.
The field is advancing rapidly. In 2023, a Boston-based team reported restoring vision in optic nerve damage models using mitochondrial transfers. Meanwhile, Japanese scientists developed a "mitochondrial drug" encapsulating organelles in biodegradable polymers for sustained release. Startups are commercializing related technologies, though regulatory approval remains years away. Critics urge caution, noting that long-term effects are unknown—could transplanted mitochondria introduce unintended mutations or disrupt cellular signaling? Rigorous clinical trials will be essential.
Beyond disease treatment, mitochondrial therapy hints at broader possibilities. Athletes might seek enhanced endurance through boosted cellular energy. Anti-aging clinics could market "mitochondrial rejuvenation" to counteract senescence. Such prospects raise ethical dilemmas about enhancement versus therapy. Yet for patients with untreatable conditions, this science represents more than hope—it’s a paradigm shift. As one researcher phrased it: "We’re not just treating symptoms anymore. We’re rebuilding the very engines of life."
The coming decade will determine whether mitochondrial transplantation transitions from lab curiosity to mainstream medicine. With billions of cells awaiting their power plant upgrades, this nanoscale surgery may well redefine what’s possible in healing the human body. For now, each successful experiment brings us closer to answering a fundamental question: If we can repair the batteries of our cells, might we finally conquer diseases once deemed incurable?

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025